The solution
Limnopharma technology
LIM21 and LIM109 molecules
Our compounds
Limnopharma develops natural phenolic derivatives, which combine neuroprotective and anti-inflammatory effects. The LIM21 and LIM109 molecules are the result of the optimization, in successive stages, of a family of 75 compounds.
In the first screening, the 18 candidates showing the best results during in vitro neuroprotection and inflammation experiments were selected.
Then, the 18 selected candidates were subjected to experiments in vivo using the zebrafish model to reassess neuroprotection and toxicity, resulting in 7 “finalist” compounds. With these compounds, AMD and RP trials in animal models (mice) were conducted.
Retinitis Pigmentosa: background image of mouse eye
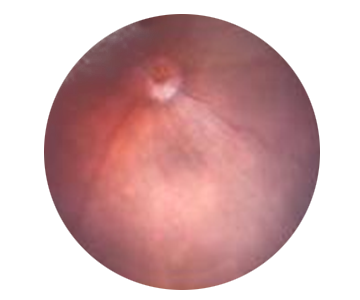
Healthy eye
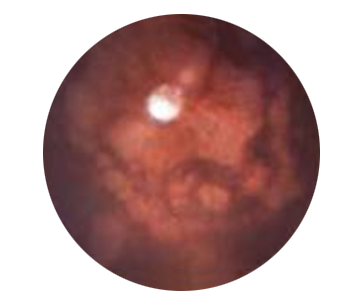
Sick eye
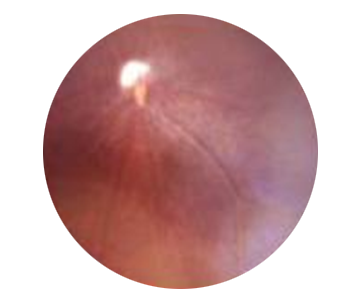
Sick eye treated with LIM21
Intellectual property
The compounds studied are protected by two patents the worldwide rights to which are the exclusive property of Limnopharma.
Limnopharma has recently entered the national phases of applications for both patents. There is freedom to operate in relation to existing related medications.
Treatments
Why do we think it is worth endorsing our treatments? Our compounds have proven effective in the treatment of both RP and dry AMD in animal models, when administered by subretinal injection and in eye drop form.
This opens up the possibility of developing topical treatments (such as eye drops or ointment), which could, in turn, foster their long-term viability as non-aggressive or invasive forms of treatment administration in patients and therefore with little or no risk to the eye.
Additionally, in contrast to most molecules currently tested in clinical trials – based on gene therapy or biological molecules -, the compounds proposed by Limnopharma are small and derive from natural products similar to traditional drugs.
It is important to remember that there is currently no treatment for either dry AMD patients or the vast majority of RP patients. Therefore, for approximately 180 million people in the world, the treatment offered by Limnopharma would be the only one available.
Our business model
The company will follow a highly virtual business model, outsourcing most validation and manufacturing activities to expedite the development of its first products and reduce capital requirements. Given the magnitude of the initial investment required, the sources of financing will come from more or less specialized private investment funds, as well as public investment and subsidies.
Limnopharma’s main objectives are:
- The manufacture of the drug from the active ingredients.
- Preclinical non-regulatory and regulatory validation.
- Approval for the clinical phases and the completion of phases I and/or I and II.
At this validation point, Limnopharma will seek opportunities to partially or totally sub-license the rights to the development and marketing of its products to large multinational pharmaceutical companies with a significant presence in the ophthalmology market.
In this way, it will take advantage of its experience and capacity in clinical trials, regulatory approval and manufacturing, as well as its distribution networks to introduce the product on the market, etc. The company’s revenue stream will consist of sub-license fees, payments by development, regulatory and marketing milestones, and ongoing royalties from drug sales.

New therapies for retinal diseases.
Treatments for Age-Related Macular Degeneration (AMD) and Retinitis Pigmentosa (RP)
Headquarters
Edif Reina María,
Carretera Bailén-Motril, S/ N
Parc 102,
18210 Peligros, Granada
